비만환자의 펜터민 복용에 따른 체중과 심박수 변화에 대한 메타분석
Meta-analysis of Change in Weight and Heart Rate for Phentermine in Obesity
Article information
Abstract
목적
식욕억제제인 펜터민의 투여에 따른 체중과 심박수 변화를 출판된 문헌을 토대로 메타분석을 시행하여 평가하고자 한다.
방법
펜터민 투여군과 위약군의 체중과 심박수 변화를 측정한 무작위배정비교임상시험을 대상으로 PubMed, EMBASE, Cochrane library와 5개의 국내 전자데이터베이스 등 모두 8개의 전자데이터베이스로부터 검색전략에 따라 문헌들이 수집되었다. 메타분석에 따른 통합효과크기의 추정은 Mantel–Haenszel법에 의한 고정효과모델이 사용되었으며, 연구 간의 이질성이 존재하는 경우 변량효과모델이 사용되었다.
결과
메타분석은 12개의 연구, 677명의 대상자에 대해 시행되었다. 펜터민의 투여에 따른 체중변화의 통합효과크기 추정치(SMD)는 -1.37 (95% 신뢰구간: -1.55, -1.19)로 통계적으로 유의하였으며, 출판연도, 국가, 펜터민의 용량, 추적관찰기간에 따른 하위그룹 분석에서 하위그룹 간 이질성은 나타나지 않았다. 펜터민의 투여에 따른 심박수 변화의 통합효과크기 추정치는 0.64 (95% 신뢰구간: 0.35, 0.92)로 통계적으로 유의하였다.
결론
펜터민의 투여는 위약에 비해 유의하게 체중을 감소시키나, 심박수를 증가시킨다.
Trans Abstract
Objectives
This study aimed to evaluate the change in weight and heart rate associated with the use of phentermine through meta-analysis based on the published literatures.
Methods
Eight electronic databases, PubMed, EMBASE, Cochrane library, and five domestic databases were used to search the literature. Randomized controlled trials that evaluated the change in weight and heart rate with the use of phentermine compared with placebo were included in this study. The fixed-effect model weighted by the Mantel–Haenszel method was used in the meta-analysis, and the random-effects model was used when heterogeneity was present.
Results
We included 12 studies comprising 677 patients. The change in weight observed with the use of phentermine (SMD=-1.37, 95% CI: -1.55, -1.19) was statistically significant compared with that observed with placebo. As per the subgroup analysis results, the change in weight by publication year, country, phentermine dosage, follow-up check was not heterogeneous. The change in heart rateobserved with the use of phentermine (SMD=0.64, 95% CI: 0.35, 0.92) was significant compared with that observed with placebo.
Conclusions
Weight loss and increased heart rate were confirmed in phentermine compared with placebo.
INTRODUCTION
Obesity is defined as a body mass index (BMI) of ≥30 kg/m2 [1]. In Korea, obesity is defined as BMI of 25 kg/m2 based on the WHO Asia-Pacific perspective for Asians [2,3]. The prevalence of obesity defined by BMI >25 kg/m2 was increased from 36.2% in 2011 to 38.9% in 2016. The prevalence rate of obesity has also been >60% in America from 2012 [4].
Obesity needs to be considered as chronic disease [5]. It was relevant to cardiovascular diseases [6], and a report showed that approximately 40% of the mortality rate in the U.S. was due to obesity or obesity-related diseases in 2000 [7]. To reduce body weight, a weight control program that includes a dietary therapy and exercise therapy needs to be tried [8], and an appetite suppressant has been generally prescribed for obesity care [9].
Phentermine, one of the popular appetite suppressants, was approved by the U.S Food and Drug Administration for short-term use for obesity in 1959 [10], and it was approved in Korea in 2004 [11]. A meta-analysis, a former study of phentermine was conducted using six published randomized controlled trials (RCTs) from 1969 to 1992, and it showed the mean weight loss of 3.6 kg in the phentermine group compared with that in the placebo group [12]. Because the previous study was conducted before the approval of the use of phentermine in Korea, the study was not intended for Koreans. In addition, the safety and efficacy of a medicine should be studied; thus, the risk and benefit should be studied [13].
It is well known that many appetite suppressants such as fenfluramine, sibutramine have a great weight loss effects, however, these agents were withdrawn from the market due to adverse events on cardiovascular system. In case of phentermine, it was also with drawn because of a lack of safety information in Europe [14]. Heart rate is an index of heart rhythm monitoring in RCT, hence, this study aimed to conduct a meta-analysis to evaluate the weight loss and increased heart rate associated with the use of phentermine.
METHODS
Inclusion criteria
This study aimed to evaluate the changes in weight and heart rate when phentermine is administered in obese individuals with no drug dependence and underlying disease compared with placebo.
Studies were eligible if they 1) were RCTs; 2) included at least one outcome, weight loss and heart rate; 3) compared between phentermine and placebo for obese patients with no underlying disease, except adjustable hypertension and diabetes; 4) provided sufficient data for analysis; 5) could be available in full-text; 6) were published in English or Korean. The studies were excluded from the analysis if the above inclusion criterion was not satisfied.
Search strategy and study selection
We searched comprehensive literature using eight electronic databases: PubMed, EMBASE, Cochrane library, KoreaMed, KMBASE, KISS, NDSL, and RISS through September 2018. The search key word, “Phentermine” in English and Korean, was entered, and MeSH term and logical operator were properly used. Studies were eventually selected after reading the titles, abstracts, and full texts. The studies for this meta-analysis were selected by two independent reviewers. If there was a difference in opinion between the two reviewers, the decision on whether to include the literature was made by consensus.
Quality assessment
The grading system developed by the Scottish Intercollegiate Guideline Network (SIGN) was used to evaluate the quality of selected literatures [15]. The studies were evaluated by two independent reviewers. If there was a difference in opinion between the two reviewers, the decision on whether to include the literature was made by consensus.
Data extraction and analysis
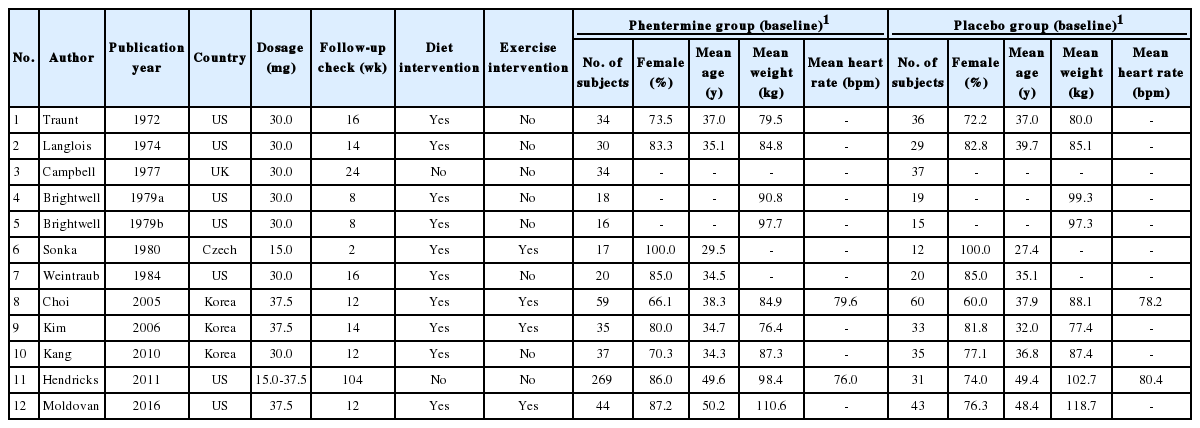
Data on the following information were extracted from each study: initial author, publication year and country, number of subjects, follow-up check, and change in weight and heart rate.
We calculated the effect size (ES) of each study as standardized mean difference (SMD) and corresponding 95% confidence intervals (95% CI) [16]. The ES of each study and overall studies was demonstrated by a forest plot graphically. The test of heterogeneity was performed using I2 statistics, and 0% to 40% may not be important, 30% to 60% reveals moderate heterogeneity, 50% to 90% is substantial heterogeneity, and 75% to 100% represents considerable heterogeneity [17]. If I2 ≥50%, it was considered heterogeneous [18]. A fixed-effect model was used if heterogeneity was not significant. If it was statistically significant, a random-effects model was performed in the subgroup analysis [19]. Publication bias was assessed by an Egger’s test and a funnel plot [20]. Statistical analyses were performed using the Stata/IC ver. 11. (StataCorp LLC, College Station, TX, USA) [21].
RESULTS
Studies included in the meta-analysis
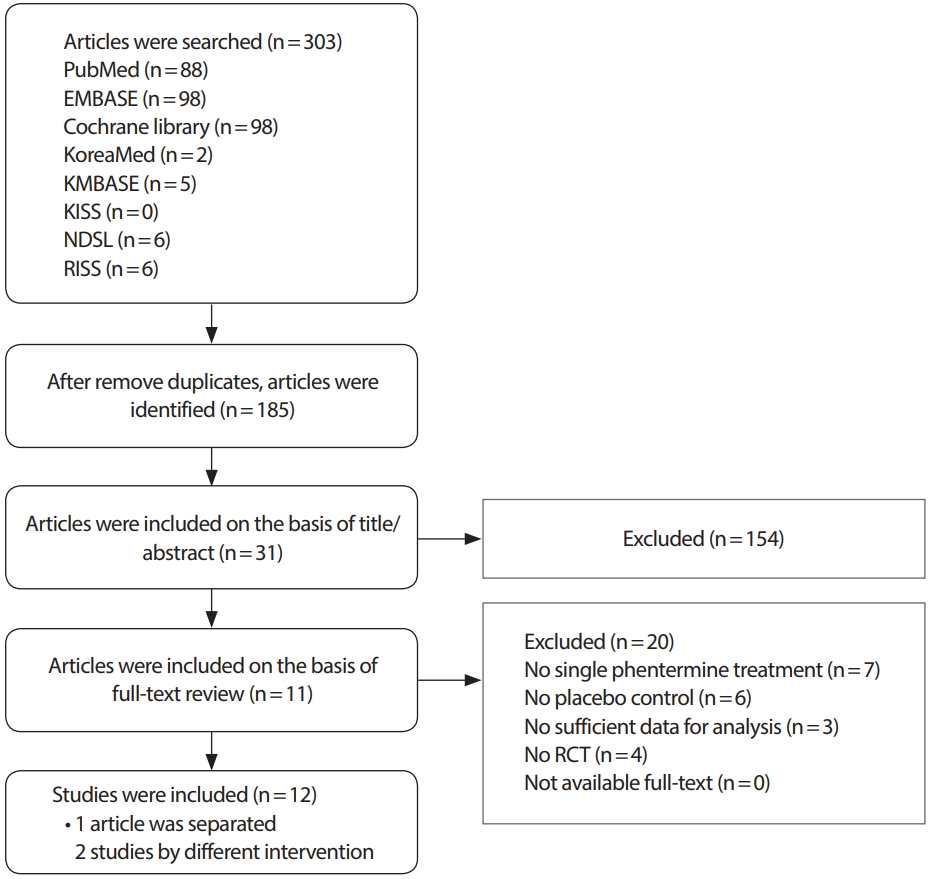
After searching the electronic databases, 185 literatures were identified, and 31 literatures were selected for review based on the title/abstract. We excluded 20 literatures for full-text review by inclusion/exclusion criteria, and 11 literatures were selected eventually. One literature was separated by two studies by different interventions [22], and 12 studies were eligible for the meta-analysis (Figure 1). The characteristic features of the studies included in the meta-analysis are given in Table 1. As a result of the quality evaluation of the literature, the evidence level of 11 literatures was 1+ (RCT with low risk of bias) and 7 with 1- (RCT with high risk of bias) and 677 subjects (400 in the phentermine group and 277 in the placebo group) were included (Table 2).
Meta-analysis of the change in weight
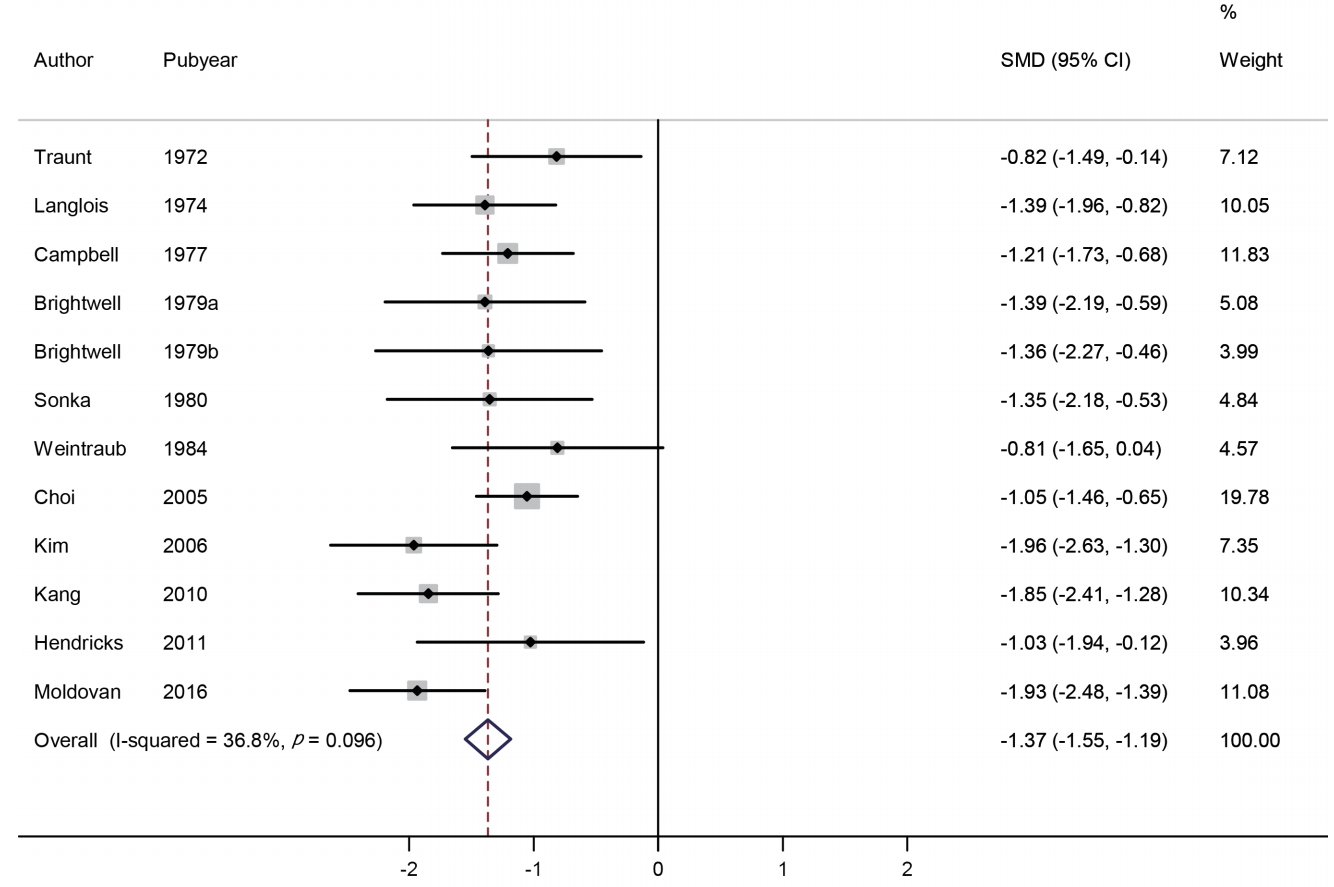
We identified 12 studies involving 677 subjects (400 in the phentermine group and 277 in the placebo group). The change in weight was compared using SMD. The effect size range of each study was -1.96 to -0.81, and the overall effect size was -1.37 (95% CI: -1.55, -1.19). Based on the meta-analysis, weight loss was greater in the phentermine group compared with the placebo group, and such differences were statistically significant (p <0.001). No significant heterogeneity was found between the studies (I2 =36.8%) (Figure 2).
Meta-analysis of the change in heart rate
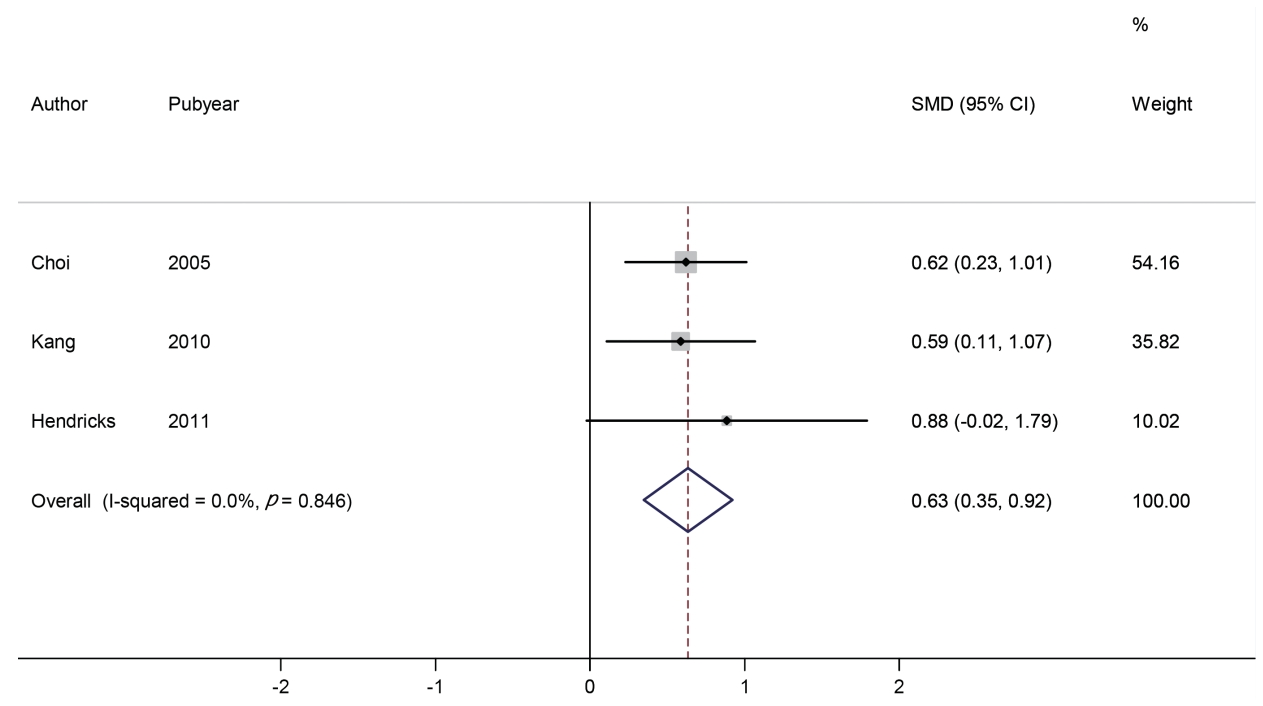
We identified three studies involving 279 subjects (188 in the phentermine group and 91 in the placebo group). The change in heart rate was compared using SMD. The effect size range of each study was 0.59 to 0.88, and the overall effect size was 0.63 (95% CI: 0.35, 0.92). Based on the analysis, the heart rate in the phentermine group was significantly increased than that in the placebo group (p <0.001). No significant heterogeneity was observed between the studies (I2 = 0.0%) (Figure 3).
Subgroup analysis
In this study, we conducted a subgroup analysis of weight change in phentermine according to the publication year, country, phentermine dosage, and follow-up check. The overall effect size of 7 studies before 2000 was -1.20 (95% CI: -1.46, -0.93), and 5 studies after 2001 was -1.58 (95% CI: -2.02, -1.13). No significant heterogeneity was observed between groups (p = 0.079). The overall effect size of 3 studies conducted in Korea was -1.58 (95% CI: -2.20, -0.97), and 9 studies conducted in foreign countries was -1.32 (95% CI: -1.55, -1.06). No significant heterogeneity was observed between groups (p = 0.479). The overall effect size of 7 studies with phentermine doses of 30 mg was -1.31 (95% CI: -1.56, -1.06) and 1 study with 15 mg was -1.35 (95% CI: -2.18, -0.53), 3 studies with 37.5 mg was -1.62 (95% CI: -2.26, -0.97), and 1 study with various amount of doses was -1.03 (95% CI: -1.94, -0.12). No significant heterogeneity was observed between groups (p = 0.714). The overall effect size of 6 studies within 12-week follow-up check was -1.46 (95% CI: -1.70, -1.22), and 6 studies over 12-week follow-up check was -1.25 (95% CI: -1.52, -0.98). No significant heterogeneity was observed between groups (p = 0.268) (Figure 4).
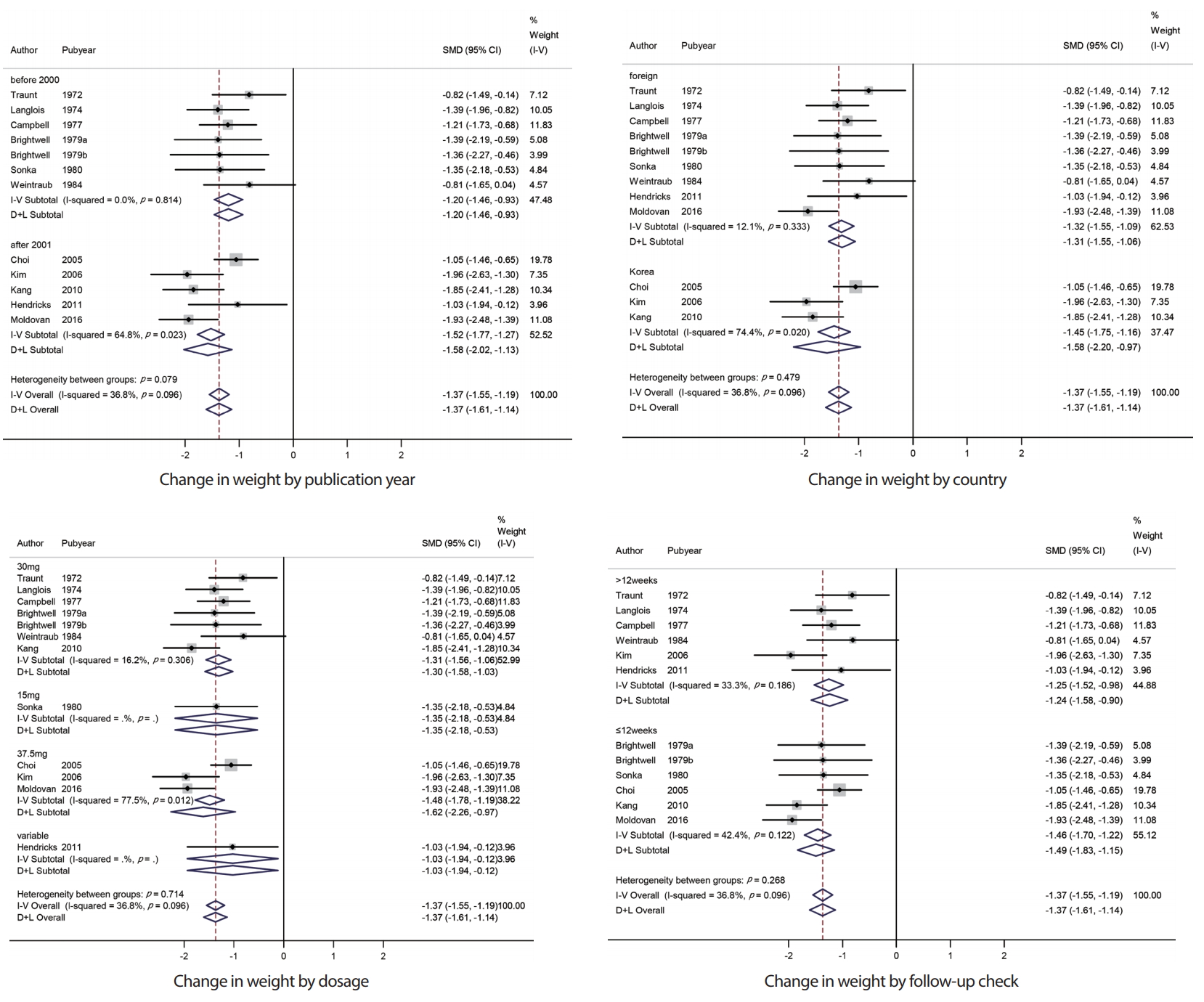
Subgroup analysis of change in weight observed with the use of phentermine and with placebo. SMD, standardized mean difference; CI, confidence interval.
A subgroup analysis of heart rate was not required because all 3 studies were published since 2001, and phentermine dosages of all 3 studies were varied. For follow-up check and country, 3 studies can be divided into 2 studies and 1 study, and it is also not required to perform subgroup analysis for only 1 study.
Evaluation of publication bias
Funnel plots were used to detect publication bias, and Egger’s test measured funnel plot asymmetry. No evidence of publication bias was identified in weight loss (p >0.1). Only 3 studies have a result of heart rate increase, thus, publication bias cannot be determined (Figure 5).
DISCUSSION
Twelve studies involving 677 subjects, including three studies published in Korea, reviewed and performed a meta-analysis to evaluate weight loss and increased heart rate associated with the use of phentermine. In the meta-analysis, the overall effect size of weight loss was -1.37 (95% CI: -1.55, -1.19), demonstrating that phentermine has an effect of weight loss. Haddock et al. [12] conducted a meta-analysis of the weight loss effect associated with the use of phentermine in 2000. However, the study published in 2002 did not include Korean studies because phentermine was approved for marketing in 2004 in Korea. Therefore, this study can be applied to the analysis of weight loss effect of phentermine in Korean.
The overall effect size of the change in heart rate associated with the use of phentermine was calculated in this study. The overall effect size of the change in heart rate was 0.64 (95% CI: 0.35, 0.92), demonstrating that the effect on heart rate associated with the use of phentermine was statistically significant compare with the placebo. The phentermine group showed a greater increase compared with the placebo group, so the study suggests that phentermine can be beneficial for weight loss, whereas the heart rate could be increased.
In this study, the meta-analysis was performed and the overall effect size could be distorted because it is excluded from the meta-analysis if the information required for calculation of the effect size such as standard deviation or standard error is not presented in the systematic review process. In addition, it is important to understand the publication bias when synthesizing studies in meta-analysis. The funnel plot cannot accurately predict the publication bias, and Egger’s test is performed when there are 10 or more individual studies. Thus, we cannot make a conclusion about the publication bias. In addition to this, there are limitations in that only literatures published in English and Korean are included in the study.
Nevertheless, to the best of our knowledge, this was the first study to perform a meta-analysis on the effect of increased heart rate associated with the use of phentermine. The result variable of the previous study was weight loss effect only. When body weight is decreased, the RR interval on electrocardiogram becomes wider, and the heart rate becomes slower [23]. However, the result of this study showed an increased heart rate in the phentermine group when body weight was decreased; hence, the trend of heart rate increase was presumed to be because of phentermine intake. Phentermine, which has a similar pharmacological activity to amphetamine, was approved for the short-term treatment of obesity [24]. Phentermine is an appetite suppressant that affects the central nervous system and releases dopamine, norepinephrine, and serotonin. Heart rate could be increased because phentermine can stimulate the central nervous system [25].
Phentermine has been associated with heart valve adverse effects in combination with fenfluramine [14]. Phentermine monotherapy is not associated with heart valve adverse events [26], and phentermine/topiramate combinations have a positive cardiovascular effect [27]. However, phentermine should not be administered to patients with pulmonary arterial hypertension, severe arterial hypertension, past or present cardiovascular disease, or cerebrovascular disease [28], long-term safety has not been confirmed [29]. Based on the studies included in the metaanalysis, the most frequent adverse drug reaction of phentermine was dry mouth and insomnia, and the incidence of the adverse reactions showed a significant increase in phentermine compared with placebo [22,30-34]. In addition, cardiovascular and neurological symptoms, such as hypertension, nervousness, depression, and palpitation, were reported [22,30,35-37].
In 2012, a young female patient with no underlying disease in Korea was reported to have died from pulmonary hypertension after taking phentermine [38]. Tachyarrhythmia is the main symptom of pulmonary hypertension. Therefore, phentermine should be used as a short-term therapy within 12 weeks based on the drug label, and arrhythmia should be carefully monitored if there are signs of heart rhythm effects, such as heart rate increase, during phentermine intake.
CONCLUSION
We conclude that weight-loss and increased heart rate are associated with the use of phentermine compared with placebo.
Notes
No potential conflict of interest relevant to this article was reported.